Trending...
- Rocket Fibre Services Growing Customer Base With netElastic Networking Software
- Jayne Williams Joins Century Fasteners Corp. Sales and Business Development Team
- Josh Stout "The Western Project"
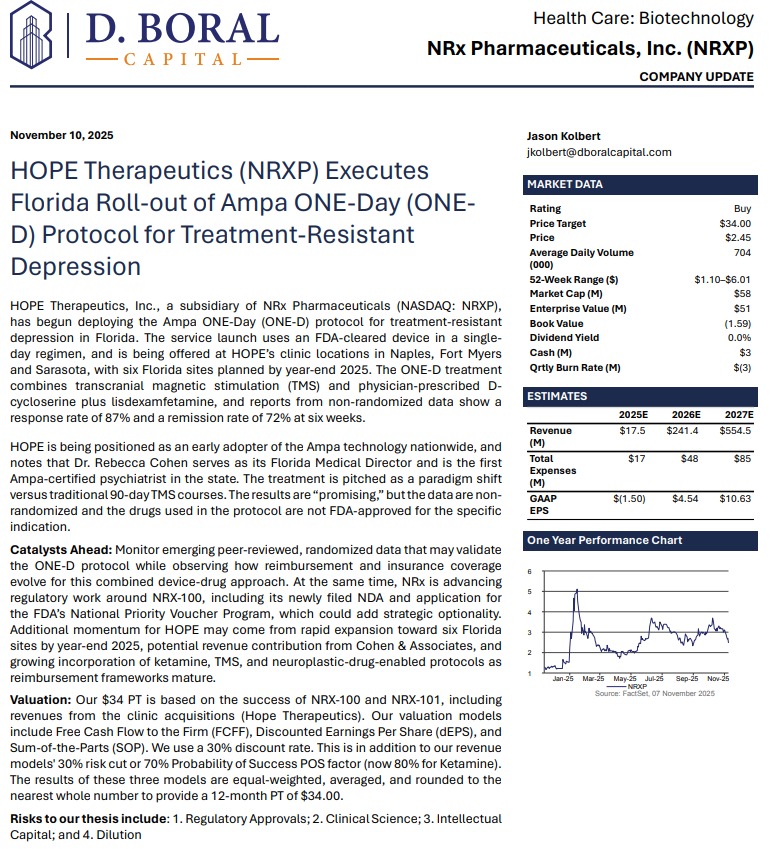
$NRXP Has Manufactured Multiple Commercial Lots of NRX-100 and KETAFREE™ with a Shelf Life of Three Years.
MIAMI - nvtip -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is rapidly emerging as one of the most compelling growth stories in mental-health therapeutics. With three revenue-generating treatment facilities now operating in Florida—and six expected by year-end—the company is entering 2026 with accelerating clinical operations, expanding market share, and advancing two FDA-directed regulatory pathways for ketamine-based therapeutics aimed at suicidal depression, one of the largest unmet needs in mental health.
Backed by strong clinical data, FDA Fast Track designations, and third-party revenue projections, NRXP appears positioned to capitalize on a rapidly expanding U.S. ketamine market currently estimated at $750 million, projected to reach $3.35 billion globally by 2034.
Adding to investor enthusiasm, analyst D. Boral issued a Buy rating and a $34 price target, citing NRXP's accelerating execution and diversified commercialization strategy.
A Mission Targeting One of America's Most Pressing Health Crises
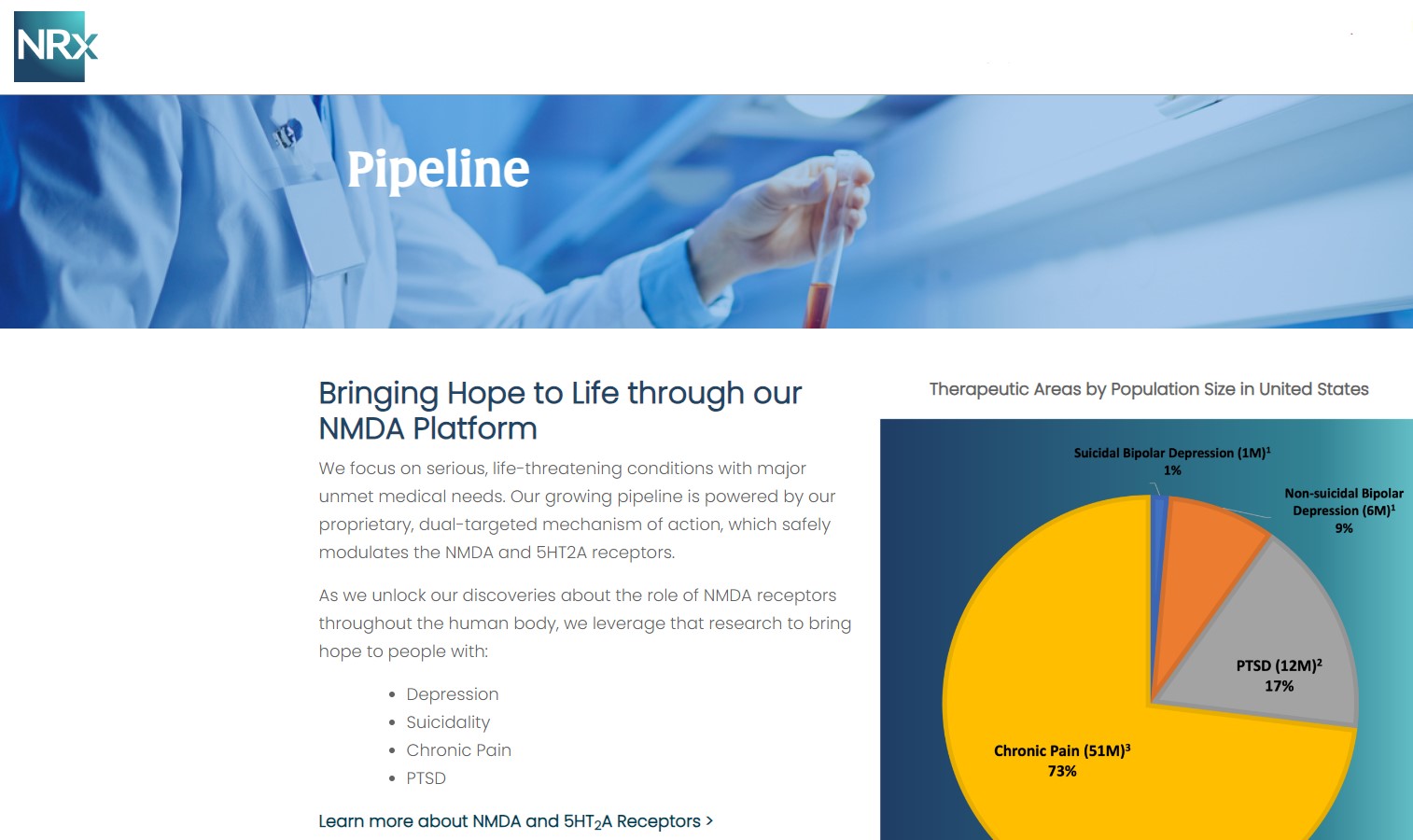
More than 13 million Americans seriously consider suicide each year (CDC). NRXP is developing next-generation therapeutics aimed directly at this crisis through its NMDA-based drug platform:
Key Pipeline Assets
NRXP is partnered with Alvogen Pharmaceuticals for the development and commercialization of NRX-101.
More on nvtip.com
A Dual-Path Strategy for NRX-100: Innovative and Generic Market Entry
NRXP's regulatory strategy is unique: it is simultaneously pursuing both an innovative NDA pathway and a generic ANDA pathway for NRX-100 and KETAFREE™.
This creates two powerful value drivers:
1. Innovative NDA for Suicidal Depression
2. ANDA for KETAFREE™ (Generic Ketamine)
With commercial lots already manufactured, NRXP is positioned for a swift commercial rollout upon approval.
HOPE Clinics: Expanding Revenue Footprint Across Florida
NRXP's HOPE Therapeutics subsidiary is advancing a scalable clinic model for ketamine and advanced TMS-based interventions.
Current & Expected Expansion
These facilities support:
Management expects increased revenue contributions from clinical operations through 2026.
Breakthrough ONE-D: First-in-Florida Deployment with Ampa Health
In November, NRXP launched patient treatment using Ampa Health's ONE-D protocol—a groundbreaking approach reporting:
ONE-D combines:
ONE-D is being deployed at HOPE clinics in Sarasota, Naples, and Fort Myers, with additional locations coming online in 2025.
More on nvtip.com
This marks a major competitive differentiator for NRXP in the mental-health treatment sector.
Financial Position: Capital Secured Through July 2026
NRXP reported securing sufficient operating capital to support its drug development programs through July 2026, complementing increasing clinical revenues.
Third-quarter results (released November 17, 2025) highlighted:
The full Q3 update and webcast are available on the NRXP investor relations site.
High-Value Licensing Opportunity
NRXP has accepted non-binding potential terms from an international pharmaceutical partner for the licensing and distribution of NRX-100. Terms include:
If finalized, this could represent a transformative revenue opportunity for the company.
Investment Outlook
With:
NRx Pharmaceuticals is attracting increased attention from both institutional and retail investors.
The recent $34 price target from D. Boral underscores growing confidence in NRXP's ability to execute on a scalable, high-margin strategy targeting one of the most underserved and urgent areas of mental health.
For More Information
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Chief Business Officer: Matthew Duffy
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Backed by strong clinical data, FDA Fast Track designations, and third-party revenue projections, NRXP appears positioned to capitalize on a rapidly expanding U.S. ketamine market currently estimated at $750 million, projected to reach $3.35 billion globally by 2034.
Adding to investor enthusiasm, analyst D. Boral issued a Buy rating and a $34 price target, citing NRXP's accelerating execution and diversified commercialization strategy.
A Mission Targeting One of America's Most Pressing Health Crises
More than 13 million Americans seriously consider suicide each year (CDC). NRXP is developing next-generation therapeutics aimed directly at this crisis through its NMDA-based drug platform:
Key Pipeline Assets
- NRX-100 (IV Ketamine):
- FDA Fast Track designation for suicidal ideation in depression, including bipolar depression.
- Multiple commercial lots produced with three-year shelf life stability.
- NDA submission expected in Q4 2025, supported by real-world patient data from more than 60,000 IV ketamine cases.
- KETAFREE™ (preservative-free ketamine):
- Pursuing approval via an ANDA (generic) pathway.
- Recently received supportive FDA correspondence confirming no major deficiencies and on track for a Q2 2026 GDUFA date.
- Addresses a ketamine market worth ~$750 million annually.
- NRX-101 (D-cycloserine + lurasidone):
- FDA-designated Investigational Breakthrough Therapy.
- Targets suicidal treatment-resistant bipolar depression, chronic pain, and potential utility in complicated UTIs.
- New real-world data suggest its active ingredient doubles the effectiveness of TMS—a potential major indication expansion.
NRXP is partnered with Alvogen Pharmaceuticals for the development and commercialization of NRX-101.
More on nvtip.com
- Tobu Group's "T-home Series" of Accommodations in Tokyo Just Opened "T-home KEI."
- Custom Wooden Token Manufacturer Celebrates 10 Years of Helping Brands Stay Top of Mind
- NaturismRE Launches the NRE Health Institute to Advance Evidence-Informed Public Health Research
- P-Wave Classics to publish Robert Bage's Hermsprong in three volumes, beginning 12 May
- Progressive Dental & The Closing Institute Partner with Zest Dental Solutions to Elevate Full-Arch Growth and Patient Outcomes
A Dual-Path Strategy for NRX-100: Innovative and Generic Market Entry
NRXP's regulatory strategy is unique: it is simultaneously pursuing both an innovative NDA pathway and a generic ANDA pathway for NRX-100 and KETAFREE™.
This creates two powerful value drivers:
1. Innovative NDA for Suicidal Depression
- Expected completion: Q4 2025.
- Includes comparative real-world data showing IV ketamine may have faster onset and greater effect than intranasal S-ketamine.
- NRXP has applied for a Commissioner's National Priority Voucher, which could accelerate FDA review.
2. ANDA for KETAFREE™ (Generic Ketamine)
- Re-filed after FDA approved NRXP's Suitability Petition for its preservative-free formulation.
- Supportive FDA feedback in November 2025 indicated no significant deficiencies.
- A Citizen Petition filed to remove toxic preservative benzethonium chloride from ketamine products could reshape the competitive landscape.
With commercial lots already manufactured, NRXP is positioned for a swift commercial rollout upon approval.
HOPE Clinics: Expanding Revenue Footprint Across Florida
NRXP's HOPE Therapeutics subsidiary is advancing a scalable clinic model for ketamine and advanced TMS-based interventions.
Current & Expected Expansion
- 3 operational facilities in Florida
- 6 additional facilities planned by year-end
These facilities support:
- NRX-100 Expanded Access Program
- Ketamine-based clinical services
- ONE-D single-day TMS depression treatments
- Veterans, first responders, and active-duty military populations
Management expects increased revenue contributions from clinical operations through 2026.
Breakthrough ONE-D: First-in-Florida Deployment with Ampa Health
In November, NRXP launched patient treatment using Ampa Health's ONE-D protocol—a groundbreaking approach reporting:
- 87% response rate
- 72% remission
- Achieved in one day, instead of traditional 90-day TMS schedules
ONE-D combines:
- Ampa's FDA-cleared TMS device
- D-cycloserine (NRX-101 active ingredient)
- Lisdexamfetamine
ONE-D is being deployed at HOPE clinics in Sarasota, Naples, and Fort Myers, with additional locations coming online in 2025.
More on nvtip.com
- CCHR: While Damaging Antipsychotics Win Approval, Proven Non-Drug Alternatives Remain Ignored
- Arcuri Group Announces Long‑Term Partnership with WakeMed Health & Hospitals to Deliver Situational Awareness and De‑escalation Training
- At 25, She Became One of the Youngest AAPI Female Founders to Win One of the World's Most Prestigious Design Awards for a Lamp That Makes You Smile
- Juego Studios Extends Full-Cycle Game Development & Outsourcing Capabilities to the UAE Market
- Unlocking The Flow With Keez MC #KeezMC @KeezMC
This marks a major competitive differentiator for NRXP in the mental-health treatment sector.
Financial Position: Capital Secured Through July 2026
NRXP reported securing sufficient operating capital to support its drug development programs through July 2026, complementing increasing clinical revenues.
Third-quarter results (released November 17, 2025) highlighted:
- Progress across all clinical and regulatory objectives
- Expanded Fast Track designation for NRX-100
- EU and NIH data enhancing regulatory strategy
- Growth of the HOPE facility platform
- Enhanced IP and formulation positioning
The full Q3 update and webcast are available on the NRXP investor relations site.
High-Value Licensing Opportunity
NRXP has accepted non-binding potential terms from an international pharmaceutical partner for the licensing and distribution of NRX-100. Terms include:
- Over $300 million in milestone payments
- Tiered double-digit royalties
If finalized, this could represent a transformative revenue opportunity for the company.
Investment Outlook
With:
- Multiple clinical catalysts approaching
- Two regulatory pathways advancing for NRX-100
- FDA Breakthrough Therapy designations
- National leadership in cutting-edge TMS + D-cycloserine therapy
- Increasing real-world data support
- A growing network of revenue-producing treatment centers
- Newly manufactured commercial drug supply
- And a strong capital runway
NRx Pharmaceuticals is attracting increased attention from both institutional and retail investors.
The recent $34 price target from D. Boral underscores growing confidence in NRXP's ability to execute on a scalable, high-margin strategy targeting one of the most underserved and urgent areas of mental health.
For More Information
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Chief Business Officer: Matthew Duffy
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on nvtip.com
- Ludex Partners With Certified Trading Card Association (CTCA) To Elevate Standards And Innovation In The Trading Card Industry
- VegasAilure Launches 'Agent Ailure': The First Agentic AI Travel Architect for Las Vegas
- Best Book Publishing Company for Aspiring Authors
- Dr. Nadene Rose Releases Moving Memoir on Faith, Grief, and Divine Presence
- Gigasoft Solves AI's Biggest Charting Code Problem: Hallucinated Property Names
- ASTI Ignites the Space Economy: Powering SpaceX's NOVI AI Pathfinder with Breakthrough Solar Technology: Ascent Solar Technologies (N A S D A Q: ASTI)
- Hiring has reached a "Digital Stalemate"—Now, an ex-Google recruiter is giving candidates the answers
- 2026 Pre-Season Testing Confirms a Two-Tier Grid as Energy Management Defines Formula 1's New Era
- Platinum Car Audio LLC Focuses on Customer-Driven Vehicle Audio and Electronics Solutions
- Postmortem Pathology Expands Independent Autopsy Services in Kansas City
- Postmortem Pathology Expands Independent Autopsy Services Across Colorado
- $38 Million in U.S. Government Contract Awards Secured Through Strategic Partner. Establishing Multi-Year Defense Revenue Platform Through 2032: $BLIS
- Mecpow M1: A Safe & Affordable Laser Engraver Built for Home DIY Beginners
- CrashStory.com Launches First Colorado Crash Data Platform Built for Victims, Not Lawyers
- Fashion Sourcing: The Wholesale Clothing Supplier That Makes Your Fashion Business Win
- Inkdnylon Earns BBB Accreditation for Verified Business Integrity
- Josh Stout "The Western Project"
- Open House Momentum Builds at Heritage at South Brunswick
- A Celebration of Visibility, Voice and Excellence: The 57th NAACP Image Awards Golf Invitational, Presented by Wells Fargo, A PGD Global Production
- Athens in Spring: A Culinary City Break That Rivals Paris and Copenhagen