Trending...
- "Micro-Studio": Why San Diegans are Swapping Crowded Gyms for Private, One-on-One Training at Sweat Society
- NewGen Business Solutions Named Oracle NetSuite 2025 Microvertical Partner of the Year
- Price Improvement on Luxurious Lāna'i Townhome with Stunning Ocean Views
$NRXP Set Up for $300 Million in Milestones on Tiered Double-Digit Royalties. NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
MIAMI - nvtip -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine. Patent expected to be Orange Book Listable.
Waiver Exemption from Paying a $4.3 Million New Drug Application Fee Under Prescription Drug User Fee Act (PDUFA).
Company On Track for Q2 2025 Completion of NDA Filing and PDUFA Date by Year End with Currently Available Resources.
Term Sheet Signed for $2.5 Million Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics.
Binding Letter of Intent with Neurospa TMS Holdings, LLC for Expansion of its Planned International Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Poised to Address Over $3 Billion Suicidal Depression Market in the US.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market
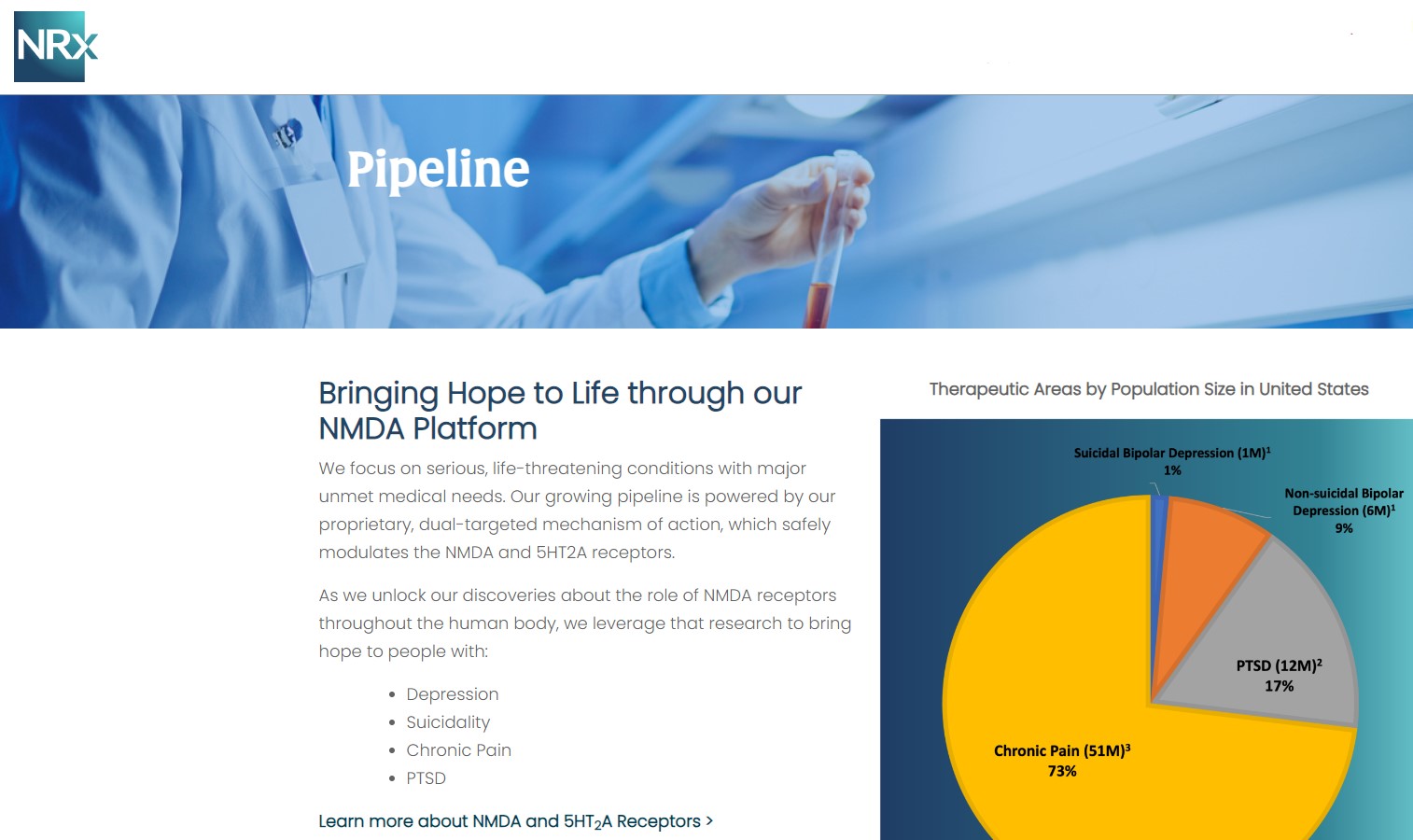
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on nvtip.com
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine
On May 5th NRXP announced the filing of a patent application for NRX-100, its preservative-free intravenous ketamine formulation for the treatment of suicidal depression. The application discloses pharmaceutical compositions, methods of treatment and methods of manufacture and currently includes twenty claims. While subject to the patent review process of the US Patent and Trademark Office, if granted, the patent would provide NRX-100 exclusivity into 2045.
This patent filing builds on the NRXP recently initiated filing of an NDA for NRX-100 and its prior Fast Track Designation, with NRX-101, from the FDA. If granted, the patent will help protect the innovation behind this formulation as NRXP advances its commercialization strategy.
"We are committed to delivering safer, more effective treatments for patients with suicidal depression," said Jonathan Javitt MD MPH, CEO of NRXP. "NRX-100 eliminates the need for benzethonium chloride, a compound with well-documented safety concerns, and reflects our belief that patients in crisis deserve therapies formulated with their long-term well-being in mind. With the recent FDA fee waiver now in place, we remain on track to complete our NDA submission this quarter — a critical step toward bringing this innovation to patients in need."
FDA Award of Filing Fee Waiver for Upcoming NRX-100 (preservative free ketamine) New Drug Application to Treat Patients with Suicidal Depression
On April 30th NRXP announced the grant of a filing fee waiver by the US Food and Drug Administration ("FDA") to exempt the Company from a $4.3 million fee to file its New Drug Application for NRX-100 (preservative-free ketamine). The waiver is granted at the discretion of the FDA to Small Business Entities and for drugs that are deemed to be necessary for Public Health. NRXP anticipates that this waiver enables the completion of its New Drug Application for NRX-100 with currently-available corporate resources. The NDA filing is anticipated by the end of the second quarter of this year (Q2 2025).
More on nvtip.com
NRXP notes recent statements by the Secretary of Health and Human Services supporting the importance of psychedelic drugs to treat severe depression and PTSD. Ketamine is believed to have a beneficial effect through its role in blocking the NMDA receptor of the brain and causing increased levels of beneficial neurotransmitters in the brain, with resulting formation of new brain cell connections (synapses).
Term Sheet for Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics
On April 3rd NRXP announced signing of a term sheet with a global medical device manufacturer, as anticipated in the Company's recently filed annual report. The investor shares NRXP subsidiary HOPE's vision of providing comprehensive interventional psychiatry treatments to patients around the world. This Investment is intended to support initiation of HOPE's network of clinics to treat suicidal depression and PTSD with ketamine, TMS and other modalities.
The Term Sheet, which is non-binding and subject to the execution of a definitive Stock Purchase Agreement, contemplates an investment of $2.5 million to purchase Series A Convertible Preferred Stock at a $50 million pre-money valuation. This investment, together with expected bank financing and current balance sheet assets, is anticipated to close concurrent with, and in support of, the closing of HOPE's recently announced clinic acquisitions.
Binding Letter of Intent with Neurospa TMS Holdings, LLC for Expansion of its Planned International Network of Interventional Psychiatry Clinics
On March 24th NRXP announced signing of a binding Letter of Intent to acquire a majority interest in Neurospa TMS Holdings, LLC. Neurospa operates six interventional psychiatry clinics on Florida's Gulf Coast and will constitute a key element of HOPE's Florida network going forward. Neurospa is revenue generating and EBITDA positive.
Neurospa leverages state-of-the-art interventional psychiatry procedures, including Ketamine Infusion Therapy, Transcranial Magnetic Stimulation (TMS), and Spravato®, augmented by traditional psychiatry and talk therapy to provide a full continuum of care for people with depression, suicidality, PTSD, anxiety, and related disorders. TMS is an FDA-approved procedure in which focused electromagnetic treatment has been demonstrated to reduce symptoms of depression. Ketamine and Spravato® are similarly known to reduce symptoms of depression and both forms of treatment are increasingly used in an additive manner.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine. Patent expected to be Orange Book Listable.
Waiver Exemption from Paying a $4.3 Million New Drug Application Fee Under Prescription Drug User Fee Act (PDUFA).
Company On Track for Q2 2025 Completion of NDA Filing and PDUFA Date by Year End with Currently Available Resources.
Term Sheet Signed for $2.5 Million Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics.
Binding Letter of Intent with Neurospa TMS Holdings, LLC for Expansion of its Planned International Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Poised to Address Over $3 Billion Suicidal Depression Market in the US.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on nvtip.com
- Guests Can Save 25 Percent Off Last Minute Bookings at KeysCaribbean's Village at Hawks Cay Villas
- Trump's Executive Order Rescheduling Cannabis: Accelerating M&A in a Multibillion-Dollar Industry
- Genuine Hospitality, LLC Selected to Operate Hilton Garden Inn Birmingham SE / Liberty Park
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- A Symphony of Support: Trang & David Hooser Champion Arts Education for Autistic Youth at NSA's Annual Gala
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine
On May 5th NRXP announced the filing of a patent application for NRX-100, its preservative-free intravenous ketamine formulation for the treatment of suicidal depression. The application discloses pharmaceutical compositions, methods of treatment and methods of manufacture and currently includes twenty claims. While subject to the patent review process of the US Patent and Trademark Office, if granted, the patent would provide NRX-100 exclusivity into 2045.
This patent filing builds on the NRXP recently initiated filing of an NDA for NRX-100 and its prior Fast Track Designation, with NRX-101, from the FDA. If granted, the patent will help protect the innovation behind this formulation as NRXP advances its commercialization strategy.
"We are committed to delivering safer, more effective treatments for patients with suicidal depression," said Jonathan Javitt MD MPH, CEO of NRXP. "NRX-100 eliminates the need for benzethonium chloride, a compound with well-documented safety concerns, and reflects our belief that patients in crisis deserve therapies formulated with their long-term well-being in mind. With the recent FDA fee waiver now in place, we remain on track to complete our NDA submission this quarter — a critical step toward bringing this innovation to patients in need."
FDA Award of Filing Fee Waiver for Upcoming NRX-100 (preservative free ketamine) New Drug Application to Treat Patients with Suicidal Depression
On April 30th NRXP announced the grant of a filing fee waiver by the US Food and Drug Administration ("FDA") to exempt the Company from a $4.3 million fee to file its New Drug Application for NRX-100 (preservative-free ketamine). The waiver is granted at the discretion of the FDA to Small Business Entities and for drugs that are deemed to be necessary for Public Health. NRXP anticipates that this waiver enables the completion of its New Drug Application for NRX-100 with currently-available corporate resources. The NDA filing is anticipated by the end of the second quarter of this year (Q2 2025).
More on nvtip.com
- Price Improvement on Luxurious Lāna'i Townhome with Stunning Ocean Views
- Nextvisit Co-Founder Ryan Yannelli Identifies Six Critical Factors for Behavioral Health Providers Evaluating AI Scribes in 2026
- CredHub and Real Property Management Join Forces to Empower Franchise Owners with Rental Payment Credit Reporting Solutions
- Leimert Park Announces Weeklong Kwanzaa Festival & Kwanzaa Parade Celebrating Black History, Culture, and Community
- Renowned Alternative Medicine Specialist Dr. Sebi and His African Bio Mineral Balance Therapy Are the Focus of New Book
NRXP notes recent statements by the Secretary of Health and Human Services supporting the importance of psychedelic drugs to treat severe depression and PTSD. Ketamine is believed to have a beneficial effect through its role in blocking the NMDA receptor of the brain and causing increased levels of beneficial neurotransmitters in the brain, with resulting formation of new brain cell connections (synapses).
Term Sheet for Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics
On April 3rd NRXP announced signing of a term sheet with a global medical device manufacturer, as anticipated in the Company's recently filed annual report. The investor shares NRXP subsidiary HOPE's vision of providing comprehensive interventional psychiatry treatments to patients around the world. This Investment is intended to support initiation of HOPE's network of clinics to treat suicidal depression and PTSD with ketamine, TMS and other modalities.
The Term Sheet, which is non-binding and subject to the execution of a definitive Stock Purchase Agreement, contemplates an investment of $2.5 million to purchase Series A Convertible Preferred Stock at a $50 million pre-money valuation. This investment, together with expected bank financing and current balance sheet assets, is anticipated to close concurrent with, and in support of, the closing of HOPE's recently announced clinic acquisitions.
Binding Letter of Intent with Neurospa TMS Holdings, LLC for Expansion of its Planned International Network of Interventional Psychiatry Clinics
On March 24th NRXP announced signing of a binding Letter of Intent to acquire a majority interest in Neurospa TMS Holdings, LLC. Neurospa operates six interventional psychiatry clinics on Florida's Gulf Coast and will constitute a key element of HOPE's Florida network going forward. Neurospa is revenue generating and EBITDA positive.
Neurospa leverages state-of-the-art interventional psychiatry procedures, including Ketamine Infusion Therapy, Transcranial Magnetic Stimulation (TMS), and Spravato®, augmented by traditional psychiatry and talk therapy to provide a full continuum of care for people with depression, suicidality, PTSD, anxiety, and related disorders. TMS is an FDA-approved procedure in which focused electromagnetic treatment has been demonstrated to reduce symptoms of depression. Ketamine and Spravato® are similarly known to reduce symptoms of depression and both forms of treatment are increasingly used in an additive manner.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on nvtip.com
- Microgaming Unveils Red Papaya: A New Studio Delivering Cutting-Edge, Feature-Rich Slots
- 5-Star Duncan Injury Group Expands Personal Injury Representation to Arizona
- The End of "Influencer" Gambling: Bonusetu Analyzes Finland's Strict New Casino Marketing Laws
- AI-Driven Cybersecurity Leader Gains Industry Recognition, Secures $6M Institutional Investment, Builds Momentum Toward $16M Annual Run-Rate Revenue
- TRIO Heating, Air & Plumbing Now Ranks #1 in San Jose
- Milwaukee Job Corps Center Hosts Alumni Day, Calls Alumni to Action on Open Enrollment Campaign
- Golden Paper Identifies Global Growth in Packaging Papers and Upgrades Its High-End Production Capacity
- Champagne, Caviar Bumps & Pole Performances — Welcome the New Year Early with HandPicked Social Club
- A New Soul Album: Heart Of Kwanzaa, 7-Day Celebration
- Allegiant Management Group Named 2025 Market Leader in Orlando by PropertyManagement.com
- Crovetti Orthopaedics Announces the Arrival of Dr. Philip Blaney, Bringing Expanded Sports Medicine Expertise to Southern Nevada
- NAFMNP Awarded USDA Cooperative Agreement to Continue MarketLink Program Under FFAB
- Costa Oil - 10 Minute Oil Change Surpasses 70 Locations with Construction of San Antonio, TX Stores — Eyes Growth Via Acquisition or Being Acquired
- LaTerra and Respark Under Contract with AIMCO to Acquire a $455M, 7-Property Chicago Multifamily Portfolio
- Record Revenue, Tax Tailwinds, and AI-Driven Scale: Why Off The Hook YS Inc. Is Emerging as a Standout in the $57 Billion U.S. Marine Market
- VSee Health (N A S D A Q: VSEE) Secures $6.0M At-Market Investment, Accelerates Expansion as Revenues Surge
- Children Rising Appoints Marshelle A. Wilburn as New Executive Director
- Fairmint CEO Joris Delanoue Elected General Director of the Canton Foundation
- Sleep Basil Mattress Co.'s Debuts New Home Page Showcasing Performance Sleep Solutions for Active Denver Lifestyles
- Bent Danholm Joins The American Dream TV as Central Florida Host