Trending...
- Kemeny, Ramp & Renaud Expands Legal Team with Attorney Baruch Kraut
- Continued Streak of Recognitions with Multiple Chambers and Partners Rankings
- IFFA 2025 Shines Bright as Mukesh Modi Welcomes Rio Rocket and Award Winner Lulu Lopez
The research study of this unique jellyfish collagen-based supplement was published in the peer-reviewed medical source, the Journal of Clinical Research and Reports.
TEMECULA, Calif. - nvtip -- Jellyfish Publication-Certified Nutraceuticals, Inc.
Gerald M. Haase, M.D.
Clinical Professor of Surgery, University of Colorado School of Medicine, Aurora, CO.
Neil E. Wolkodoff, PhD
Medical Program Director, Colorado Center for Health and Sports Science, Denver, CO.
May 16, 2025
Certified Nutraceuticals, Inc., a research and product development enterprise in Pauma Valley, California, U.S.A., announces the publication of an exciting new human clinical trial of its proprietary KollaJell™ collagen peptide formulation.
The research study of this unique jellyfish collagen-based supplement was published in the peer-reviewed medical source, the Journal of Clinical Research and Reports. The strength of this prospective, randomized trial is the distinctive utilization of a panel of brain function metrics from two robust, validated instruments, including a computerized neurocognitive test battery and an electroencephalographic (EEG) profile of voltage activation and wave phase pattern. Instead of evaluating only one or two parameters, this investigation assessed seven domains of cognitive function in 23 subjects who consumed KollaJell™ for a period of eight weeks.
More on nvtip.com
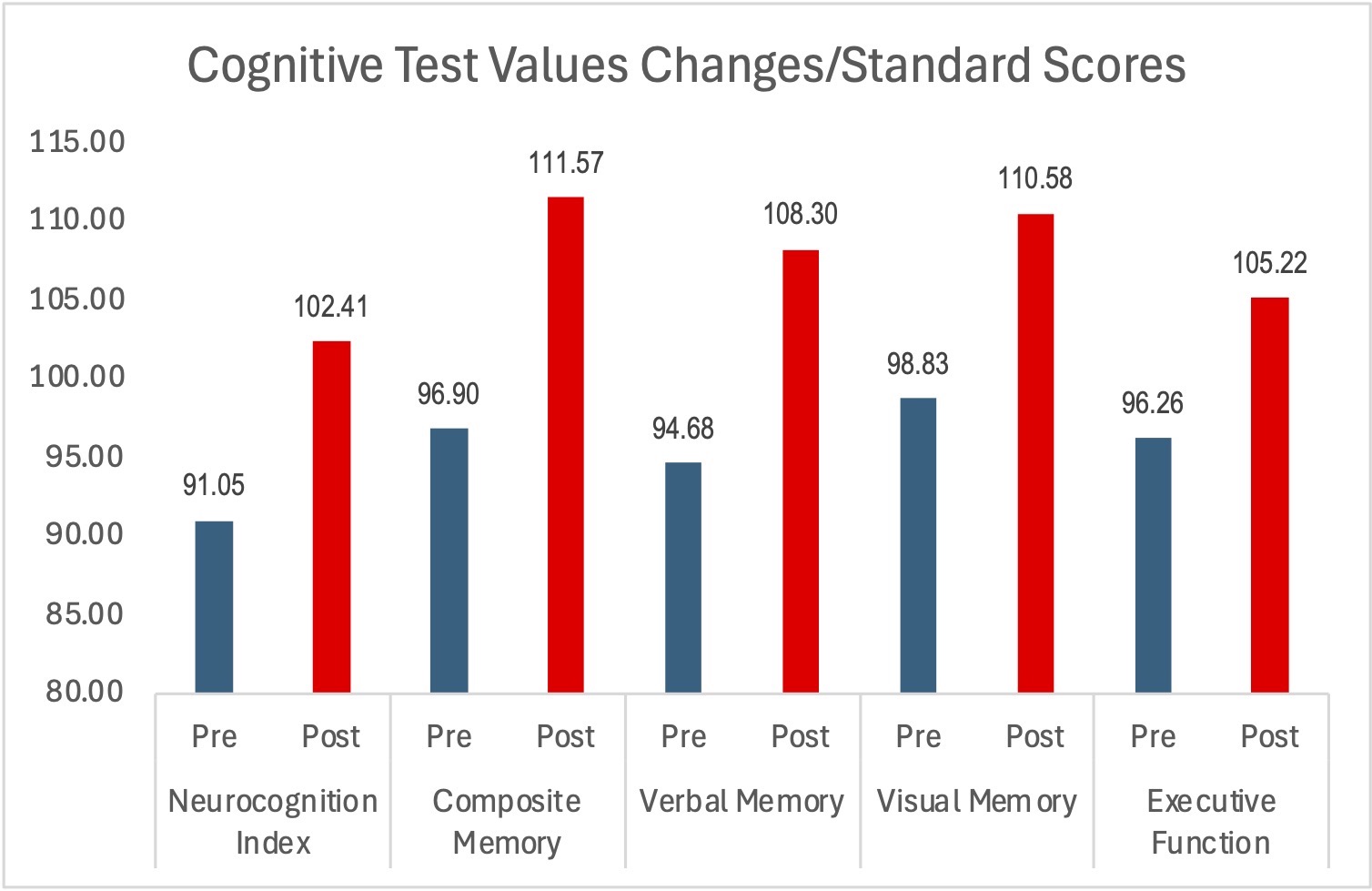
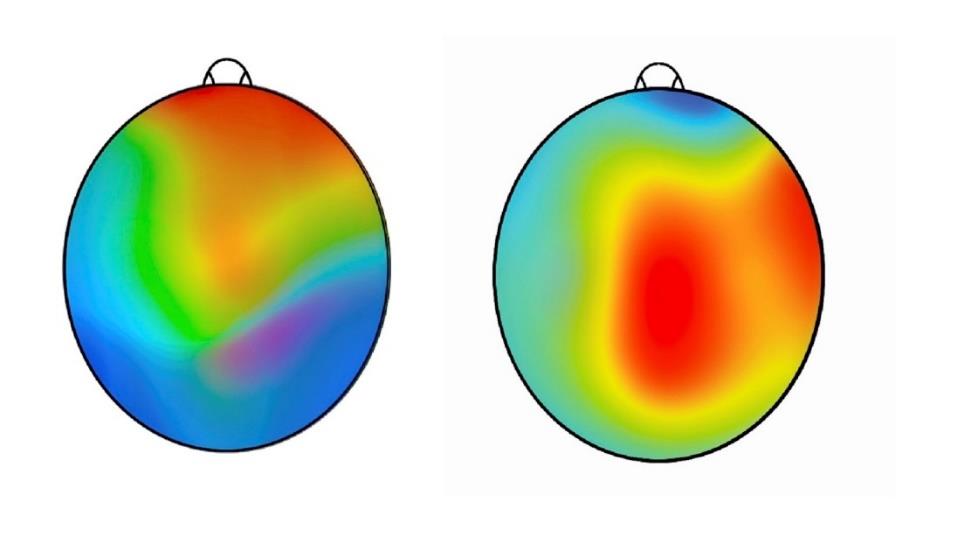
The outcomes were clinically relevant, and all were statistically analyzed and achieved statistical significance. Standard scores matched for age and gender improved in all metrics regardless of the functional status of where the subject started. The parameters included compositive memory, neurocognitive index, visual memory, executive function and verbal memory. Under EEG monitoring conditions, the mean total reaction time and the trail-making composite test times also decreased dramatically. Topographic brain images showed voltage activation in the brain regions associated with sensory input and processing function. There were no adverse effects of consuming the supplement in any subject at any time during the study.
The importance of this pilot trial is the demonstration of a particularly extensive spectrum of beneficial results from the proprietary formulation compared to competing products that, even if helpful, provide only limited effects. In addition, the use of a marine-based natural collagen was superior to the usual animal-based products and the patented extraction process provided a greater platform of collagen types and a uniquely effective amino acid composition. This human clinical trial reinforces the concept that Certified Nutraceuticals KollaJell™ is a safe and beneficial collagen supplement that supports cognitive domains and memory and is applicable to a wide audience of potential consumers, regardless of their initial brain function status.
More on nvtip.com
The actual medical study is available as a free open-access publication at https://www.auctoresonline.org/article/effects-of-a-jellyfish-collagen-based-amino-acid-supplement-on-cognitive-function-and-memory-a-pilot-investigation
For further information about the product or study, please contact:
Sara Alkayali at (951) 600-3899 or email: salkayali@certifiednutra.com
Gerald M. Haase, M.D.
Clinical Professor of Surgery, University of Colorado School of Medicine, Aurora, CO.
Neil E. Wolkodoff, PhD
Medical Program Director, Colorado Center for Health and Sports Science, Denver, CO.
May 16, 2025
Certified Nutraceuticals, Inc., a research and product development enterprise in Pauma Valley, California, U.S.A., announces the publication of an exciting new human clinical trial of its proprietary KollaJell™ collagen peptide formulation.
The research study of this unique jellyfish collagen-based supplement was published in the peer-reviewed medical source, the Journal of Clinical Research and Reports. The strength of this prospective, randomized trial is the distinctive utilization of a panel of brain function metrics from two robust, validated instruments, including a computerized neurocognitive test battery and an electroencephalographic (EEG) profile of voltage activation and wave phase pattern. Instead of evaluating only one or two parameters, this investigation assessed seven domains of cognitive function in 23 subjects who consumed KollaJell™ for a period of eight weeks.
More on nvtip.com
- Initial Order Received from Vietnamese Maritime Security and Defense Services for Advanced Video Compression Solution: RMX; Stock Symbol: RMXI
- Keebos Launches New Universal Pearl Strap That Attaches to Any Phone Case
- Arbonne's 2025 Global Training Conference Celebrates 45 Years of Innovation and Community with Groundbreaking Product Launches
- Work 365 Launches Certified Provider Integration (CPI) Program to Help Distributors and CSPs Navigate Microsoft's Evolving Ecosystem
- Five Aster Awards! Fusion Marketing Group Brings Home Big Wins in 2025!
The outcomes were clinically relevant, and all were statistically analyzed and achieved statistical significance. Standard scores matched for age and gender improved in all metrics regardless of the functional status of where the subject started. The parameters included compositive memory, neurocognitive index, visual memory, executive function and verbal memory. Under EEG monitoring conditions, the mean total reaction time and the trail-making composite test times also decreased dramatically. Topographic brain images showed voltage activation in the brain regions associated with sensory input and processing function. There were no adverse effects of consuming the supplement in any subject at any time during the study.
The importance of this pilot trial is the demonstration of a particularly extensive spectrum of beneficial results from the proprietary formulation compared to competing products that, even if helpful, provide only limited effects. In addition, the use of a marine-based natural collagen was superior to the usual animal-based products and the patented extraction process provided a greater platform of collagen types and a uniquely effective amino acid composition. This human clinical trial reinforces the concept that Certified Nutraceuticals KollaJell™ is a safe and beneficial collagen supplement that supports cognitive domains and memory and is applicable to a wide audience of potential consumers, regardless of their initial brain function status.
More on nvtip.com
- AI changes cryptocurrency market trading, TWL Miner launches new 1-day contract, giving away over $1 million
- A Global First: NaturismRE™ Launches the Global Urgency Index
- 14th CryptoSuper500 Report Releases: Bitcoin's Evolution into a Global Supercomputer
- Ross Toohey named Managing Director in Chesterfield, Missouri
- High Profile Gateway Race Events With Strong Brand Exposure Plus Sponsorship in Female Motorsports; $100 Million Financing Unlocked: Lottery.com
The actual medical study is available as a free open-access publication at https://www.auctoresonline.org/article/effects-of-a-jellyfish-collagen-based-amino-acid-supplement-on-cognitive-function-and-memory-a-pilot-investigation
For further information about the product or study, please contact:
Sara Alkayali at (951) 600-3899 or email: salkayali@certifiednutra.com
Source: Certified Nutraceuticals, Inc.
0 Comments
Latest on nvtip.com
- New Cook Book Release! Tagalog Language: Let's Cook Filipino!
- Discover the Joy of Language Learning with Tagalog Language: Learn Tagalog the Fun and Easy Way!
- DivX Enhances AVI Playback Resources; Simplifies Guide to Playing AVI Files with DivX Software
- Patrick Aloni Joins Historic Gold and Copper Discovery in Argentina with Multimillion-Dollar Stake
- Edtech Startup Young Commanders Launches 'Visionaries Without Sight' Collection Celebrating Blind and Visually Impaired Historical Figures
- Info-Tech LIVE 2025 Day 2 Highlights: Bold Strategies for AI, IT Leadership, and Digital Disruption
- Goldstar Rehabilitation Celebrates 15 Years of Early Intervention Across Southeastern PA
- United States Congressional Candidate Peter Coe Verbica Unveils 25-Point Federal Plan to Help Make California Affordable Again
- D8Averse Launches D8Acapture: Disruptive Mobile-First App Transforms Utility Pole Data Collection
- Experience Trembling Firsthand with the New AgeMan® Tremor Simulator
- Wise Business Plans Leads the Way in Investor-Ready Business Plans and Pitch Decks for Startups
- Mauro Schnaidman named as Managing Director in Miami, Florida
- Continued Streak of Recognitions with Multiple Chambers and Partners Rankings
- Anern Shines at SOLAR AFRICA Kenya with Solar Lithium Battery Storage Technology
- Last Call - Submit Your Proposal for the 2025 OpenSSL Conference in Prague
- Robert Michael & Co. Launches New Real Estate Website to Serve Central Florida Homebuyers and Sellers
- Tacotarian Named to Fast Casual's Top 100 Movers & Shakers List
- IFFA 2025 Shines Bright as Mukesh Modi Welcomes Rio Rocket and Award Winner Lulu Lopez
- Kemeny, Ramp & Renaud Expands Legal Team with Attorney Baruch Kraut
- WOA Entertainment Group Unveils Independent No.1's: Breakthrough Artists II —A Celebration of Indie Excellence