Trending...
- New Article by Roy J. Meidinger – Examines Hidden Hidden Healthcare Kickbacks - 104
- Stop Monitoring Start Preventing: Phinge to Challenge Identity Monitoring Platforms With Netverse App-less Platform, AI & Hardware-based Verification
- Zero-Trust Architecture: NJTRX Addresses 60% of U.S. Investors' Custody Security Concerns
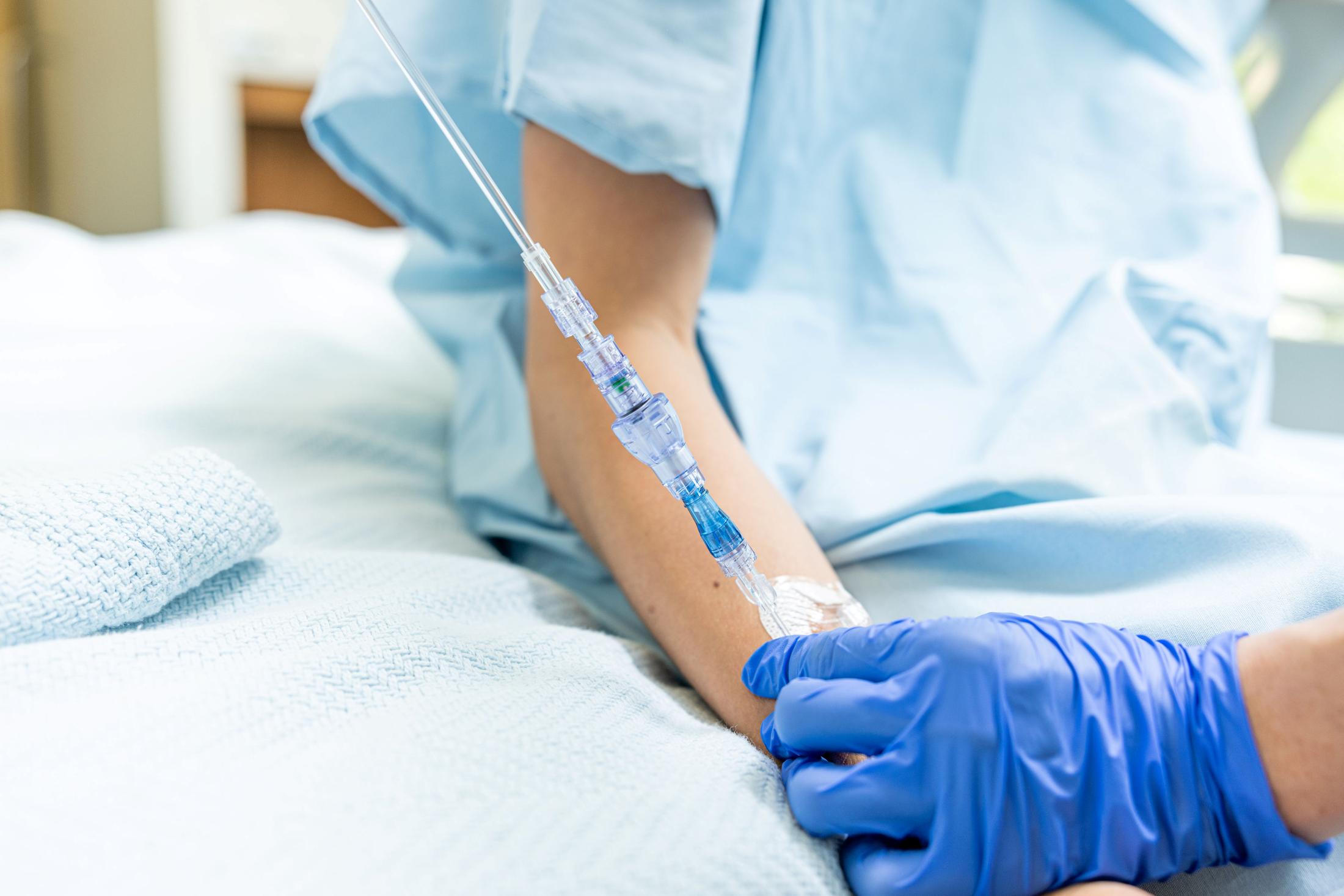
FAYETTEVILLE, Ark. - nvtip -- Lineus Medical has obtained CE Mark certification for its flagship product, SafeBreak® Vascular, the only breakaway device for IV lines clinically proven to reduce multiple IV complications.1 With this certification, SafeBreak Vascular is now cleared for use across the European Union. This approval expands SafeBreak Vascular's clearance from 5 countries to 32, spanning across more than three continents. Lineus Medical is actively partnering with distributors across Europe to expand hospital access to SafeBreak worldwide.
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on nvtip.com
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on nvtip.com
- Inflation Rebounds Under Tariff Shadow: Wall Street Veteran Kieran Winterbourne Says Macro Signals Matter More Than Market Sentiment
- Phinge Stores To Debut Immersive Netverse Experience: Interact with Magnetically Connectable Smartphones, Tablets, Gaming Systems & More
- Mensa Foundation's New Science Program Encourages Hands-On Discovery
- Golden Paper Introduces TAD Hand Towel Technology, Ushering in a New Era of Premium Tissue Quality
- Turn Your Laptop Into Tablets: Textbook by Phinge Will be a Wireless, Integrated Multi-&-Triple Screen Laptop-&-Tablets-in-One for Use With Netverse
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
Source: Lineus Medical
Filed Under: Technology
0 Comments
Latest on nvtip.com
- Are You Ready? Phinge Magnetically Attachable 360° Folding Smartphones-to-Tablets Gateway to Netverse App-less Rewards-based Platform & AI are Coming
- Blogging Pioneer Sherry Bennett Celebrates 29 Years Online - Sharing the Secrets Behind Her 7-Figure Blog Empire
- Koplon Dentistry Elevates Implant Expertise with Advanced CE Course
- i2 Group Acquisitions and Investments in Innovations Deliver 40% Increase in Year-on-Year Bookings
- Netverse Verified Platform and AI Will Safeguard the Future of Humanity, Says Phinge CEO
- New Book Release: The Tree That Could Not Change
- BayWa r.e. Solar Trade and WHES Announce Distribution Partnership for the European Market: Delivering Smarter Energy Storage
- Never Charge Your Smartwatch Again: Phinge's Smartwatches with Interchangeable/Chargeable Battery Bezels to be a Gateway to Netverse App-less Platform
- Fleet Mining Cloud Mining Platform — Latest Guide: Making Bitcoin Mining Safer and More Convenient
- Keebos Launches Crossbody Cases for Every iPhone 17 Model
- WOA Crypto redefines cloud Bitcoin mining, offering free access and instant profit opportunities
- Pepperdine University Malibu, California and Community Partners Recognized with 2025 ReadyCommunities Partnership National Service Award
- NBA Overachievers: OddsTrader Reveals Which Teams Will Exceed Expectations in 2025-26
- Crypto Betting Odds 2025: Bookmakers Review Analyzes Market Predictions and Year-End Price Lines
- The OpenSSL Corporation and the OpenSSL Foundation Celebrate the Success of the Inaugural OpenSSL Conference in Prague
- TKL Group's New Factory Commences Production, Pioneering A New Era In Global Heavy Duty Truck Parts
- Regulated Crypto Exchange TZNXG Addresses Core US Market Challenges with Compliance-First Infrastructure
- GitKraken Launches Insights to Help Engineering Leaders Quantify AI Impact and Improve Developer Experience
- ZapperBox NextGen TV Gateway Receiver Now Testing Support For Secure Whole-Home Content Distribution
- Life as a Dog: P-Wave Press Brings Readers a Heartwarming Memoir of Love, Laughter and Companionship