Trending...
- Sahit Muja Launches Trillion-Dollar Green Revolution with 10 Groundbreaking Cryptos
- Free and Low-Cost Dental Care Now Available in London Through the Canadian Dental Care Plan (CDCP)
- Snell & Wilmer Partner John Delikanakis Elected Board Trustee of the African American Archive
Devices not cleared by the FDA are being sold in the USA! Buying non-fda cleared device can cause injury. Spa Sciences launches LEXI after receiving FDA clearance for effective treatment. IPL devices are considered medical devices and have rigorous requirements. Spa Sciences launches LEXI IPL device for permanent hair removal after FDA clearance bringing customers a lifetime of hair removal in a single device. Get safe and affordable permanent hair removal in the comfort of your home with LEXI.
PORT ST. LUCIE, Fla. - nvtip -- Spa Sciences, the #1 facial skin device brand in the United States, announces it has been granted FDA clearance for its newest IPL device LEXI for a permanent home-use hair removal solution.
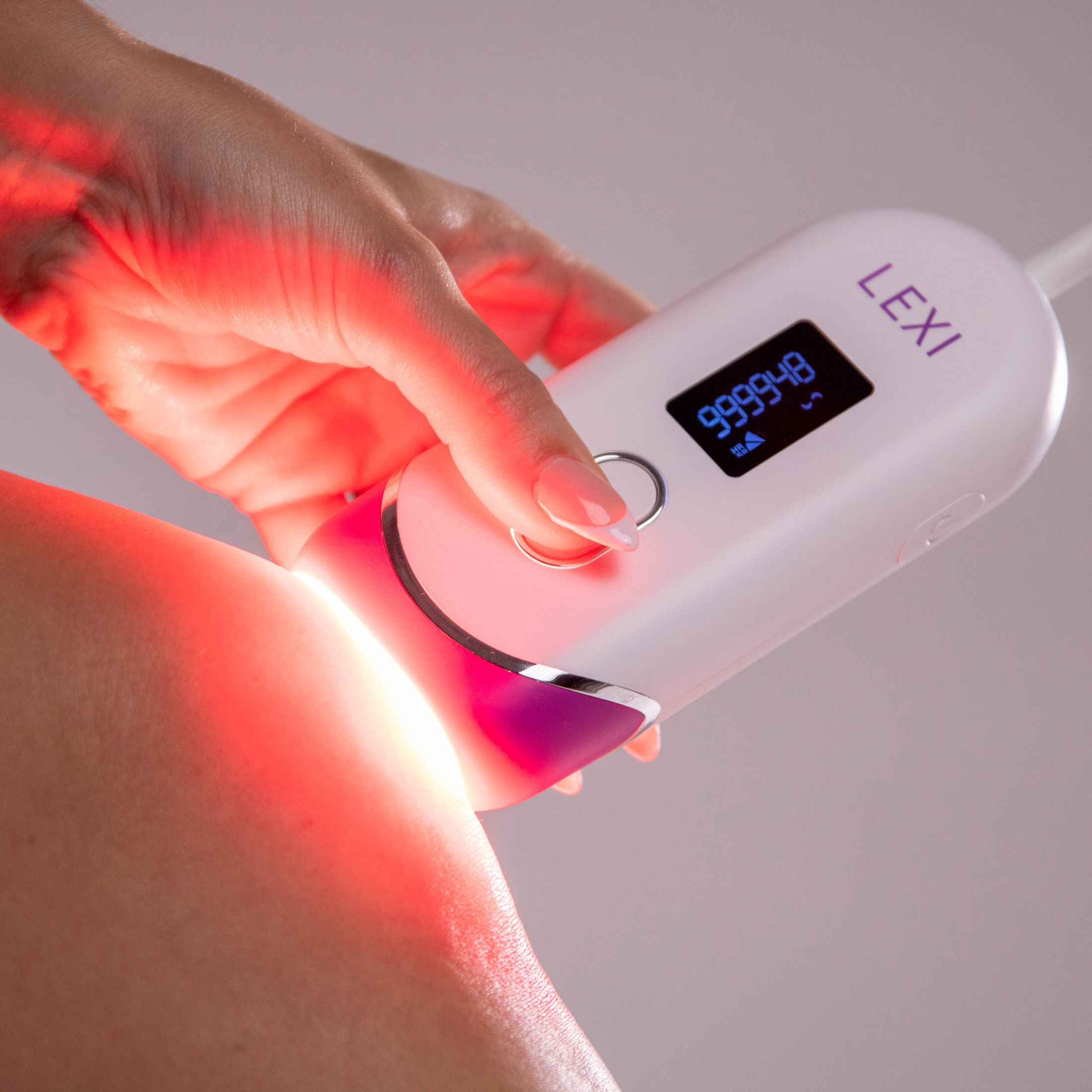
IPL stands for intense pulsed light. It works similarly to laser hair removal, only it's way easier and safer to do at home. LEXI is a handheld device that targets unwanted hairs at the root and destroys them with light pulses without burning or damaging your skin. It stops hair from growing back for long-term hair reduction from the comfort of your home.
More on nvtip.com
An IPL is considered a medical device which requires FDA authorization before it can be sold legally in the U.S. "There are a number of IPL devices to choose from on Amazon and elsewhere the vast majority of which are not legally cleared by the FDA, so consumers need to be careful when purchasing an IPL and make sure the product they select from Amazon is FDA approved or cleared as required by law," said co-founder and president Michael Friend. "Our LEXI IPL hair removal device has undergone rigorous trials to ensure that the device is safe, effective and FDA compliant so our customers can purchase LEXI with confidence, knowing treatment will be effective and safe."
About LEXI by Spa Sciences
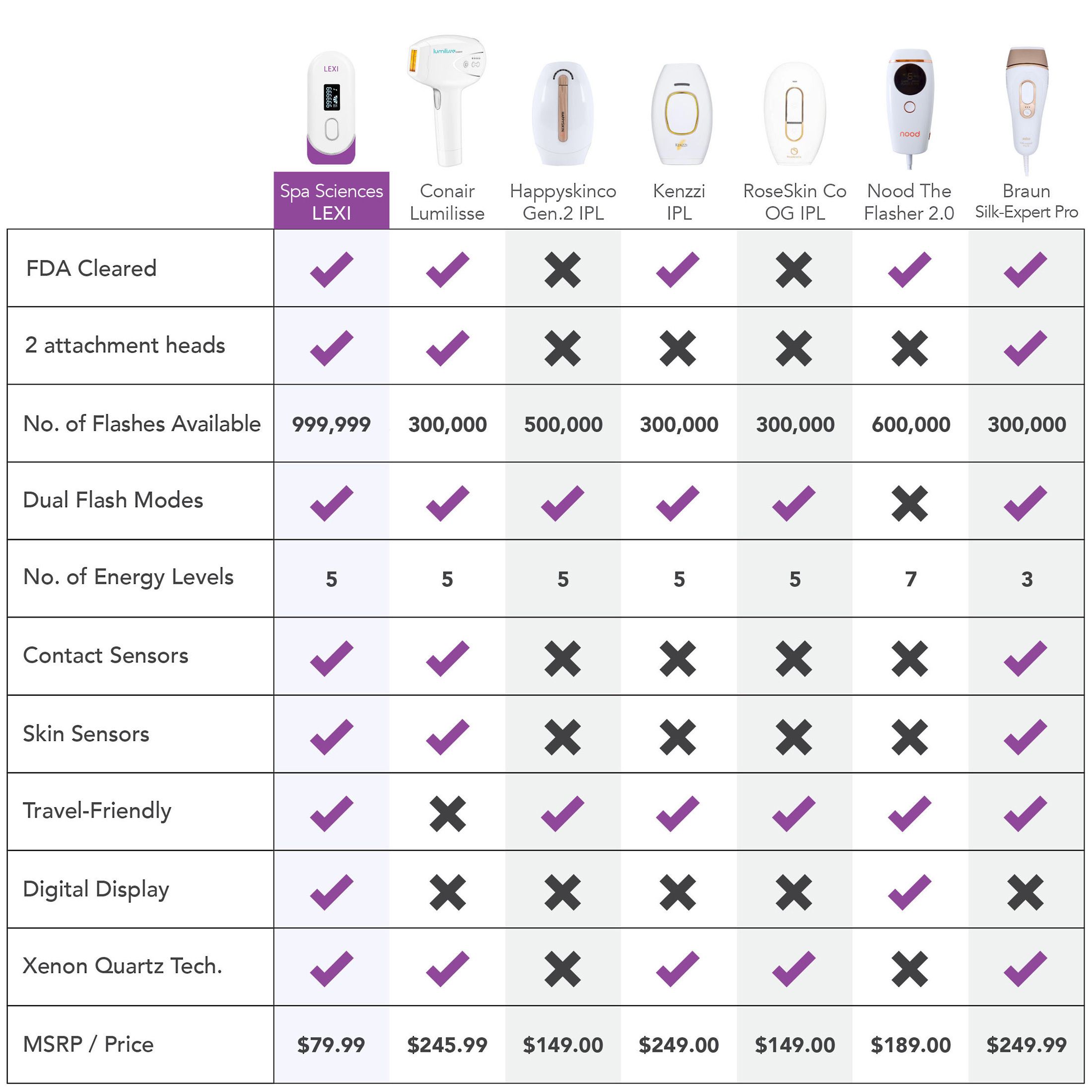
LEXI is a noninvasive Intensive Pulse Light therapy device, FDA cleared for the removal of unwanted hair long-term or permanently. Boasting an impressive 1,000,000 flashes capacity - enough for a lifetime of hair removal - the cutting-edge hair removal technology of LEXI is incredibly effective at visibly reducing or eliminating hair growth and features auto flash, skin sensors, and digital screen for easy and safe hair removal.
More on nvtip.com
https://youtu.be/VbmrMc1VxQE
About Spa Sciences
Spa Sciences is the #1 facial skin device brand in the U.S., according to Nielsen, a global leader in audience measurement, data, and analytics. Spa Sciences is a leading expert in developing and innovating affordable skin technologies that have redefined at-home skin care treatments to bring the spa experience home through advanced beauty & wellness devices.
IPL stands for intense pulsed light. It works similarly to laser hair removal, only it's way easier and safer to do at home. LEXI is a handheld device that targets unwanted hairs at the root and destroys them with light pulses without burning or damaging your skin. It stops hair from growing back for long-term hair reduction from the comfort of your home.
More on nvtip.com
- Snell & Wilmer Partner John Delikanakis Elected Board Trustee of the African American Archive
- NBA Champion Lamar Odom Launches Anti-Addiction Meme Coin, Ushering in a Disruptive Innovation in Web3
- Aureli Construction Sets the Standard for Seamless Home Additions in Greater Boston
- Prism Bank's Delene Gilbert Speaks at 2025 Financial Brand Forum
- ScreenPoints Puts Film Investors in the Credits—and in the Money With New FinTech Platform
An IPL is considered a medical device which requires FDA authorization before it can be sold legally in the U.S. "There are a number of IPL devices to choose from on Amazon and elsewhere the vast majority of which are not legally cleared by the FDA, so consumers need to be careful when purchasing an IPL and make sure the product they select from Amazon is FDA approved or cleared as required by law," said co-founder and president Michael Friend. "Our LEXI IPL hair removal device has undergone rigorous trials to ensure that the device is safe, effective and FDA compliant so our customers can purchase LEXI with confidence, knowing treatment will be effective and safe."
About LEXI by Spa Sciences
LEXI is a noninvasive Intensive Pulse Light therapy device, FDA cleared for the removal of unwanted hair long-term or permanently. Boasting an impressive 1,000,000 flashes capacity - enough for a lifetime of hair removal - the cutting-edge hair removal technology of LEXI is incredibly effective at visibly reducing or eliminating hair growth and features auto flash, skin sensors, and digital screen for easy and safe hair removal.
More on nvtip.com
- Pathways to Adulthood Conference May 17 at Melville Marriott Honoring NYS Assembly Member Jodi Giglio, Suffolk County Legislator Nick Caracappa
- Adster Techologies awarded US Patent for breakthrough innovation in reducing latency in Ad Serving
- Robert Fabbio Inducted into the Austin Technology Council Hall of Fame
- Dr. Dennis Jackson Brings Non-Operating Orthopaedic Sports and Emergency Medicine Expertise to Crovetti Orthopaedics and Sports Medicine
- Cybersecurity is Protecting Your Personal Information and Your Portfolio
https://youtu.be/VbmrMc1VxQE
About Spa Sciences
Spa Sciences is the #1 facial skin device brand in the U.S., according to Nielsen, a global leader in audience measurement, data, and analytics. Spa Sciences is a leading expert in developing and innovating affordable skin technologies that have redefined at-home skin care treatments to bring the spa experience home through advanced beauty & wellness devices.
Source: Spa Sciences
0 Comments
Latest on nvtip.com
- ImagineX, in Collaboration with Qualys, Launches New mROC Services to Transform Enterprise Cyber Risk Management
- Ditch Micromanagement: New Leadership Book for Results-Driven, Accountability-Based Teams
- Jay Tapp was named Managing Director in British Columbia
- Hubei Heavy Equipment Makes a Striking Appearance at CIMT and Competes with International Brands
- 20 Patents Issued Worldwide, Cementing Company Leadership. First Ever Cable-Free 12-Lead ECG: HeartBeam, Inc. (Stock Symbol: BEAT)
- NASDAQ Uplisting for Higher Market Exposure and Wide Corporate Benefits to AI Boosted Marketing Company On Track Towards $1 Billion Revenue by 2027
- Congressional Men's Health Caucus Shows Bipartisan Consensus and Focus on Prevention, Mental Health, and Closing the Lifespan Gap
- DuoKey, Axiomtek and Blue Edge Network Partner to Enhance Smart Cities with Privacy-Preserving Urban Safeguarding and Fleet Management
- Mix Master AI Bartender Launches Web Platform, Revolutionizing Cocktail Creation
- Evolving Gaming Regulations: New Insights From Info-Tech Research Group Shows How IT Leaders Can Strengthen Compliance and Reduce Risk
- Austin Keen Joins WakeFX RopePal as Official Brand Ambassador
- Bonelli Systems Expands Managed IT Services Nationwide, Leveraging Microsoft Azure Expertise
- $4.3 Million Patent Application Waiver Fee Granted by FDA on New Drug Application Fee for Treatment Addressing Suicidal Depression & PTSD: NRX Pharma
- Whistleblower Claims Dental Patient Deaths Likely Due to Book Ban
- xREnergy up as much as +3,094,634% on first day listed on the XRP Ledger. Ticker : $XRE
- Psychiatry's Legacy of Racism and Coercion Highlighted in Restraint Deaths
- New Book 'Cybersecurity Leadership' Guides SME Leaders to Make Smart, Strategic Security Decisions
- "Stop scrolling and start watching" - Beloved film recommendation site Criticker gets a major makeover
- Green Energy Solar Expands with New Offices in Port St. Lucie, West Palm Beach, and Orlando
- Events by Dubsdread Expands Services to The Venue at Lake Lily